Delving into the fascinating world of draw valine at physiological pH, we embark on a journey to unravel the intricate dance of ionization and its profound impact on this essential amino acid. As we explore the chemical structure of valine and its behavior at varying pH levels, we will uncover the significance of ionization in shaping the structure and function of proteins and other biological molecules.
Prepare to be captivated as we delve into the different ionization states of valine, deciphering the pKa values and predominant forms that govern its behavior. The titration curve of valine will unveil the stages of protonation and deprotonation, revealing the isoelectric point where the zwitterion form takes center stage.
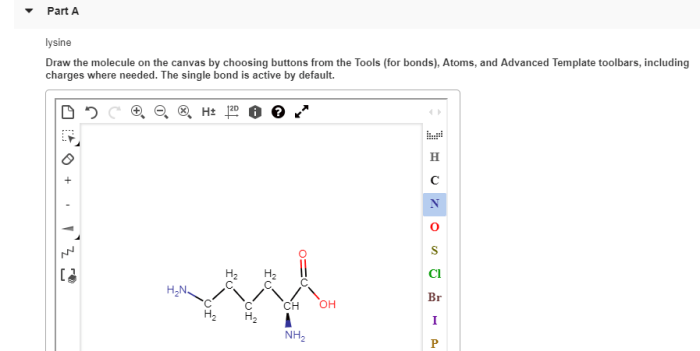
Draw Valine at Physiological pH
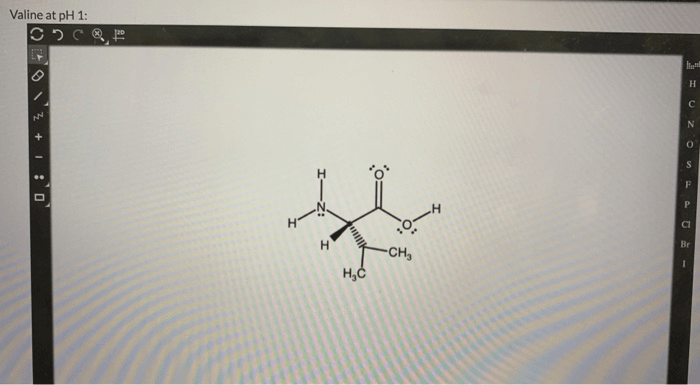
Valine is an amino acid with the chemical formula C 5H 11NO 2. At physiological pH (around 7.4), the carboxylic acid group (-COOH) of valine is deprotonated, while the amino group (-NH 2) is protonated. This results in a net charge of +1 for the valine molecule.
Chemical Structure
The chemical structure of valine at physiological pH is shown below:

The carbon atoms are numbered 1 to 5, with the carboxylic acid group at C1 and the amino group at C2. The side chain of valine is a branched hydrocarbon chain, which gives valine its hydrophobic properties.
Ionization States of Valine
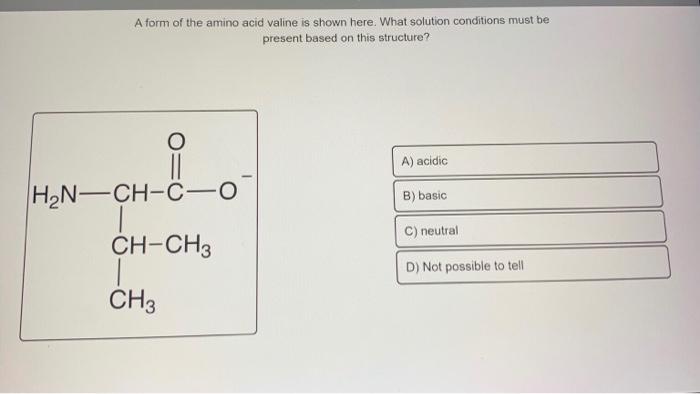
Valine, like other amino acids, can exist in different ionization states depending on the pH of its surroundings. Understanding these ionization states is crucial for comprehending the chemical behavior and properties of valine in biological systems.
Drawing valine at physiological pH involves understanding its chemical structure and properties. To delve deeper into this topic, I recommend tuning into for a segment of a radio show that explores this subject in detail. By examining valine’s behavior under physiological conditions, we can gain valuable insights into its role in various biological processes.
pKa Values and Predominant Forms
Valine has three ionizable groups: the amino group, the carboxylic acid group, and the side chain. The ionization of these groups occurs at specific pH values known as pKa values.
| Ionizable Group | pKa | Predominant Form at pH 7.4 |
|---|---|---|
| Amino group | 9.6 | -NH3+ |
| Carboxylic acid group | 2.3 | -COO– |
| Side chain | 10.6 | -CH(CH3)2 |
At physiological pH (around 7.4), the amino group of valine is predominantly protonated (-NH 3+), while the carboxylic acid group is deprotonated (-COO –). The side chain remains neutral (-CH(CH 3) 2).
Ionization States and pH Ranges
The ionization states of valine change as the pH of the environment varies. Here’s a summary of the ionization states and their respective pH ranges:
- pH < 2.3:Both the amino and carboxylic acid groups are protonated (-NH 3+and -COOH).
- pH 2.3- 9.6: The carboxylic acid group is deprotonated (-COO –), while the amino group remains protonated (-NH 3+).
- pH 9.6- 10.6: The amino group is deprotonated (-NH 2), while the carboxylic acid group remains deprotonated (-COO –).
- pH > 10.6:Both the amino and side chain groups are deprotonated (-NH 2and -CH(CH 3) 2–).
Understanding the ionization states of valine is essential for comprehending its chemical reactivity and biological functions.
Titration Curve of Valine
The titration curve of valine, a neutral amino acid, displays a characteristic pattern when titrated with a strong base (e.g., NaOH) or a strong acid (e.g., HCl). The curve provides insights into the ionization states of valine and its buffering capacity at different pH values.
Titration with a Strong Base
As a strong base is added to a solution of valine, the following stages of titration can be observed:
- Stage 1:The initial addition of the base protonates the carboxylic acid group (-COOH) of valine, converting it to the zwitterionic form (+NH3-CH(CH3)-COO-). This stage occurs at a pH below the first pKa (pKa1) of valine (2.32).
- Stage 2:Further addition of the base deprotonates the ammonium group (-NH3+) of the zwitterion, forming the neutral form of valine (NH2-CH(CH3)-COO-). This stage occurs at a pH between pKa1 and the second pKa (pKa2) of valine (9.62).
- Stage 3:At higher pH values beyond pKa2, the hydroxide ions from the strong base deprotonate the hydroxyl group (-OH) of the neutral valine, forming the fully deprotonated form of valine (NH2-CH(CH3)-COO-). This stage results in a sharp increase in pH.
Titration with a Strong Acid
When a strong acid is added to a solution of valine, the following stages of titration occur:
- Stage 1:The initial addition of the acid protonates the ammonium group (-NH3+) of valine, converting it to the cationic form (+NH3-CH(CH3)-COOH). This stage occurs at a pH above pKa2.
- Stage 2:Further addition of the acid protonates the carboxylic acid group (-COOH) of the cationic valine, forming the neutral form of valine (NH2-CH(CH3)-COOH). This stage occurs at a pH between pKa2 and pKa1.
- Stage 3:At lower pH values below pKa1, the hydrogen ions from the strong acid protonate the hydroxyl group (-OH) of the neutral valine, forming the fully protonated form of valine (+NH3-CH(CH3)-COOH). This stage results in a sharp decrease in pH.
Titration Curve Graph
The titration curve of valine can be represented graphically, with pH on the y-axis and the volume of base or acid added on the x-axis. The curve typically shows three equivalence points, corresponding to the complete protonation or deprotonation of the three ionizable groups in valine.
The isoelectric point (pI) of valine, where the net charge of the molecule is zero, is at a pH of 5.96. This value is approximately the average of the two pKa values of valine.
Zwitterion Formation and Isoelectric Point
At its isoelectric point (pI), valine exists predominantly in its zwitterion form. The zwitterion is a dipolar species with both positive and negative charges, resulting from the protonation of the amino group and the deprotonation of the carboxylic acid group.
The net charge of the zwitterion is zero, hence its name.
Significance of Isoelectric Point
The isoelectric point plays a crucial role in biochemical processes. Proteins, composed of amino acids like valine, exhibit different solubilities and electrophoretic mobilities at different pH values. At their isoelectric points, proteins have zero net charge and are least soluble.
This property is utilized in protein purification techniques, such as isoelectric focusing, where proteins are separated based on their isoelectric points.
Applications of Valine’s Ionization Properties
The ionization properties of valine play crucial roles in biological systems, particularly in protein structure and function. Understanding these properties enables researchers to investigate the behavior of valine within proteins and its impact on overall protein characteristics.
Protein Structure and Stability, Draw valine at physiological ph
Valine’s ionization behavior influences protein structure by affecting the electrostatic interactions between amino acid residues. At physiological pH, valine exists predominantly as a zwitterion, contributing to the overall charge distribution of the protein. This charge distribution influences protein folding, stability, and interactions with other molecules.
For example, in the protein hemoglobin, valine residues contribute to the stability of the protein’s tertiary structure. The ionization state of valine residues can alter the electrostatic interactions within the protein, potentially affecting its overall stability and function.
Enzyme Catalysis
Valine’s ionization properties also play a role in enzyme catalysis. Enzymes are proteins that facilitate chemical reactions in biological systems. The ionization state of valine residues can influence the active site of an enzyme, affecting its ability to bind to substrates and catalyze reactions.
In the enzyme chymotrypsin, for instance, the ionization state of a valine residue in the active site is crucial for substrate binding. The protonation state of this valine residue alters the electrostatic environment of the active site, influencing the enzyme’s catalytic activity.
Commonly Asked Questions
What is the physiological pH range?
The physiological pH range typically refers to the pH values found in living organisms, which is generally between 6.8 and 7.4.
Why is understanding valine’s ionization behavior important?
Understanding valine’s ionization behavior is crucial because it influences the overall charge and solubility of the amino acid, which in turn affects protein structure, function, and interactions with other molecules.
What is the isoelectric point of valine?
The isoelectric point of valine is approximately 5.96, which represents the pH at which the amino acid carries no net charge.