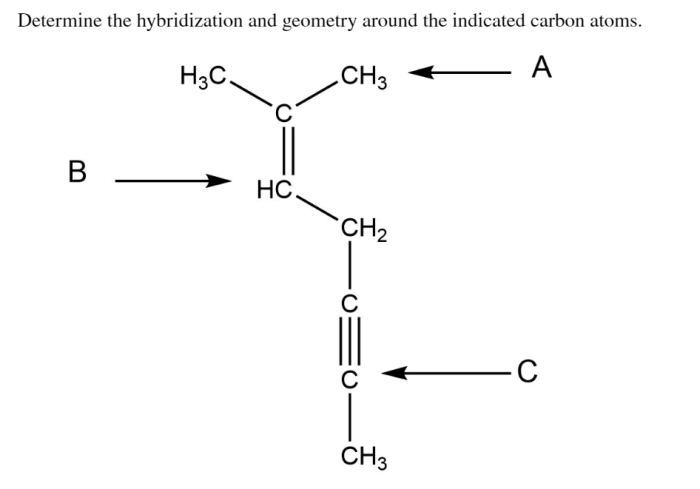
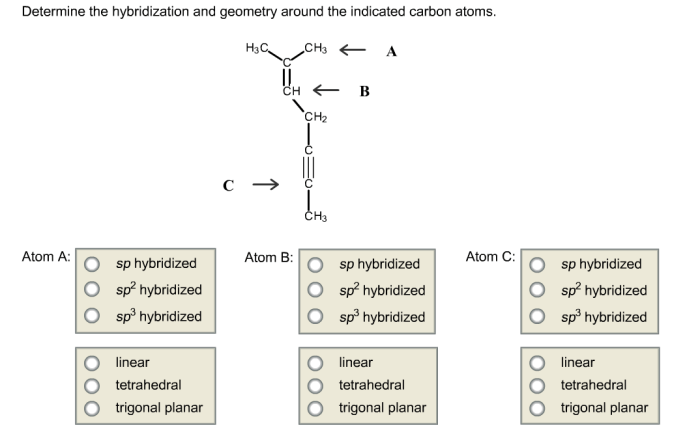
Determine the hybridization and geometry around the indicated carbon atoms – Determining the hybridization and geometry around the indicated carbon atoms is a crucial aspect of understanding the structure and properties of organic molecules. This knowledge enables chemists to predict the reactivity, polarity, and other characteristics of molecules, which is essential in fields such as organic chemistry, biochemistry, and materials science.
In this comprehensive guide, we will explore the concepts of hybridization and molecular geometry, discuss the steps involved in determining these properties for carbon atoms, and highlight their applications in various scientific disciplines.
Hybridization of Carbon Atoms
Hybridization is a fundamental concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. It plays a crucial role in determining the molecular geometry and properties of compounds.
Electron Configuration and Hybridization States
Carbon atoms can exhibit three main hybridization states: sp, sp2, and sp3.
- sp Hybridization:One s orbital and one p orbital combine to form two sp hybrid orbitals, each with a linear geometry and 180° bond angle.
- sp2 Hybridization:One s orbital and two p orbitals combine to form three sp2 hybrid orbitals, arranged in a trigonal planar geometry with 120° bond angles.
- sp3 Hybridization:One s orbital and three p orbitals combine to form four sp3 hybrid orbitals, arranged in a tetrahedral geometry with 109.5° bond angles.
Relationship to Sigma and Pi Bonds
The hybridization state of carbon atoms influences the number and type of bonds they can form.
- sp hybridized carbon atoms form one sigma bond and one pi bond.
- sp2 hybridized carbon atoms form three sigma bonds and one pi bond.
- sp3 hybridized carbon atoms form four sigma bonds.
Geometry Around Carbon Atoms
The hybridization state of carbon atoms directly determines the molecular geometry around them.
Linear Geometry (sp Hybridization)
sp hybridized carbon atoms have a linear geometry, with two bonds extending in opposite directions.
Trigonal Planar Geometry (sp2 Hybridization)
sp2 hybridized carbon atoms have a trigonal planar geometry, with three bonds arranged in a plane at 120° angles.
Tetrahedral Geometry (sp3 Hybridization)
sp3 hybridized carbon atoms have a tetrahedral geometry, with four bonds arranged in a three-dimensional tetrahedron with 109.5° angles.
Influence on Molecular Shape and Properties
The hybridization of carbon atoms influences the shape and properties of molecules.
- Linear molecules have a rod-like shape.
- Trigonal planar molecules have a flat, triangular shape.
- Tetrahedral molecules have a three-dimensional shape.
- Hybridization also affects bond lengths, bond strengths, and molecular polarity.
Determining Hybridization and Geometry: Determine The Hybridization And Geometry Around The Indicated Carbon Atoms
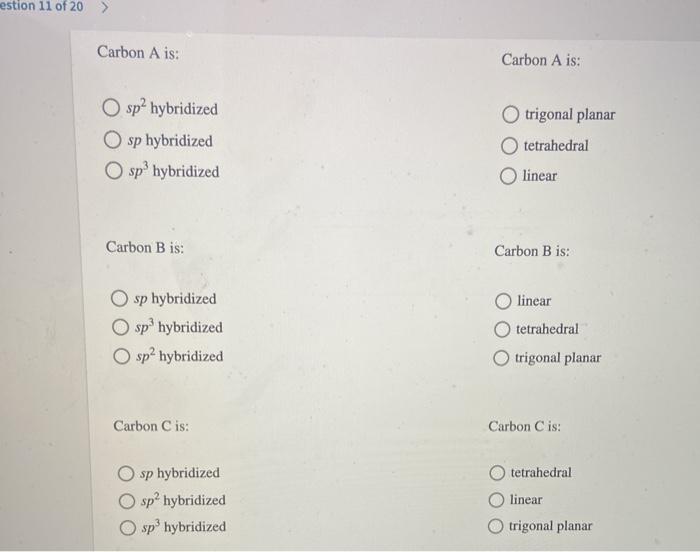
Steps for Determining Hybridization and Geometry
- Count the number of sigma and pi bonds formed by the carbon atom.
- Use the table of hybridization states to determine the hybridization of the carbon atom.
- Based on the hybridization, determine the molecular geometry around the carbon atom.
Molecular Orbital Theory and Valence Electron Counting, Determine the hybridization and geometry around the indicated carbon atoms
Molecular orbital theory and valence electron counting can be used to predict the hybridization and geometry of carbon atoms.
- Molecular Orbital Theory:Explains how atomic orbitals combine to form molecular orbitals, which can be used to determine the hybridization state of carbon atoms.
- Valence Electron Counting:Involves counting the number of valence electrons in a molecule to determine the hybridization state and geometry of carbon atoms.
Examples
- CH4 (Methane):Four sigma bonds, sp3 hybridization, tetrahedral geometry.
- C2H4 (Ethylene):Two sigma bonds and one pi bond, sp2 hybridization, trigonal planar geometry.
- C2H2 (Acetylene):One sigma bond and two pi bonds, sp hybridization, linear geometry.
Applications of Hybridization and Geometry
Predicting Reactivity and Polarity
Understanding hybridization and geometry is crucial for predicting the reactivity and polarity of molecules.
- sp hybridized carbon atoms are more reactive and form stronger bonds.
- sp2 hybridized carbon atoms are less reactive and form weaker bonds.
- sp3 hybridized carbon atoms are the least reactive and form the weakest bonds.
Applications in Organic Chemistry, Biochemistry, and Materials Science
Hybridization and geometry have wide-ranging applications in various fields:
- Organic Chemistry:Understanding the hybridization and geometry of carbon atoms is essential for predicting the structure and reactivity of organic molecules.
- Biochemistry:The hybridization and geometry of carbon atoms play a crucial role in the structure and function of biological molecules, such as proteins and DNA.
- Materials Science:Hybridization and geometry influence the properties of materials, such as strength, conductivity, and optical properties.
Clarifying Questions
What is hybridization?
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies.
How does hybridization affect molecular geometry?
The hybridization of carbon atoms determines the number and arrangement of sigma and pi bonds, which in turn influences the molecular geometry.
How can I determine the hybridization and geometry of a carbon atom?
The hybridization and geometry of a carbon atom can be determined using molecular orbital theory and valence electron counting.